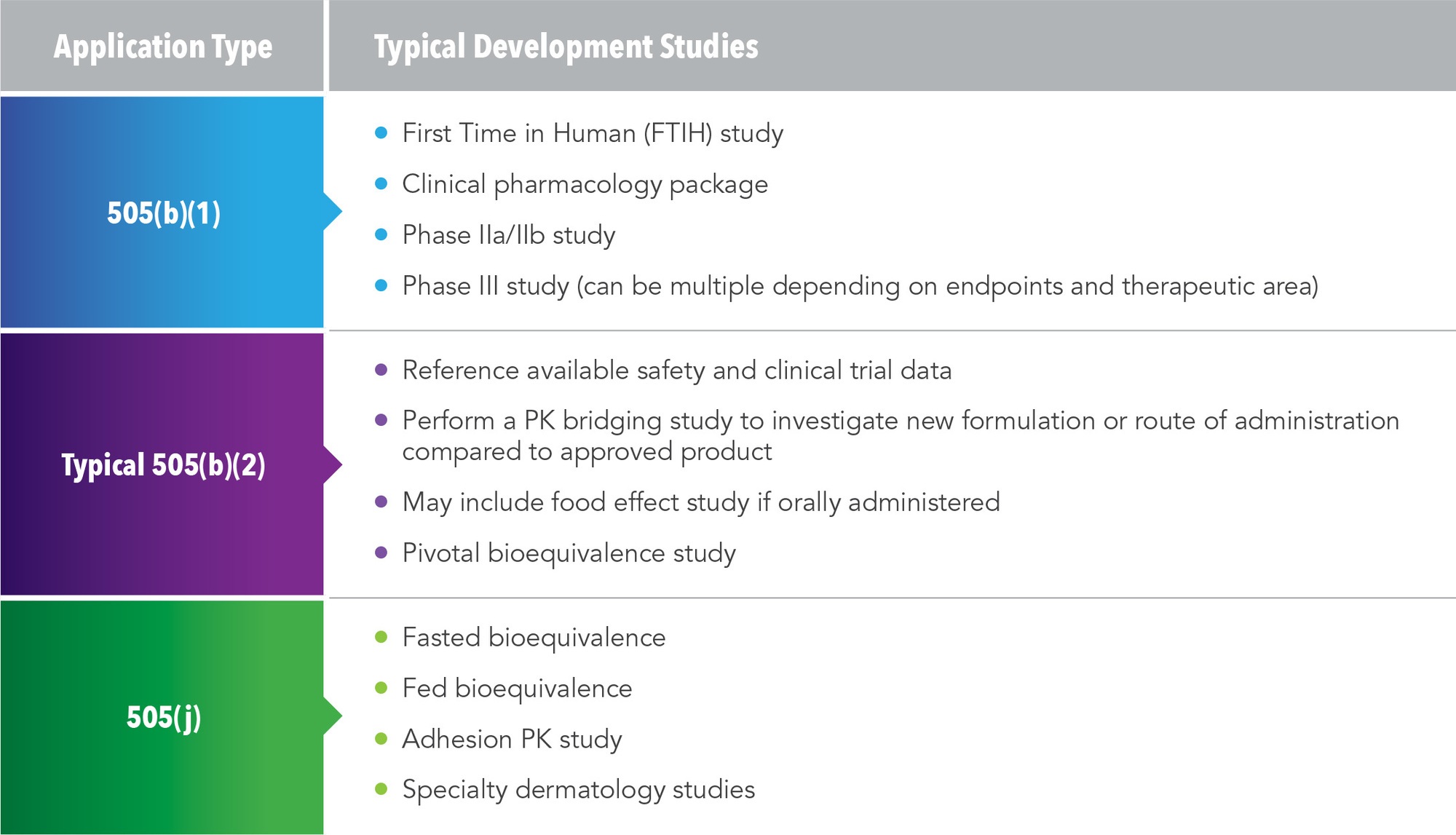
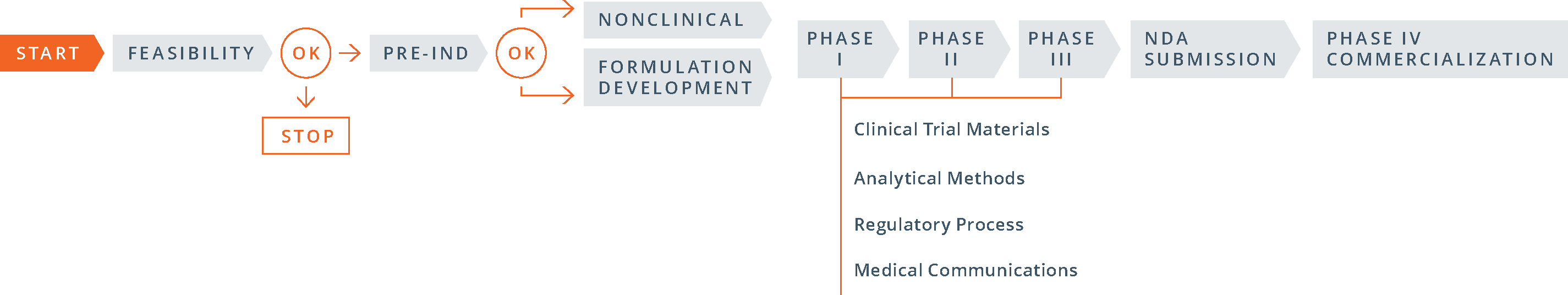
Postmarketing Studies and Clinical Trials—Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act

USA - Determining Whether to Submit an ANDA or a 505(b)(2) Application - Guidance for Industry - RIS.WORLD

Postmarketing Studies and Clinical Trials—Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act | HartmannWillner
Sixth Annual Report on Delays in Approvals of Applications related to Citizen Petitions and Petitions for Stay of Agency Action